
Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions.

And with respect to carbon a lone pair is added. This gives the oxygen atom a total of eight valence electrons (6 electrons of the lone pairs and 2 electrons of the a single O-C bond). To satisfy the octet rule for the oxygen atom, we place three lone pairs of electrons around the oxygen atom. The remaining 2 electrons (1 lone pair) are drawn as a lone pair on carbon.
#CO CARBON MONOXIDE LEWIS STRUCTURE FULL#
In the case of carbon monoxide, the oxygen atom needs six more electrons to achieve a full octet, so, 3 electron pairs are added. The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons (or two electrons for hydrogen).
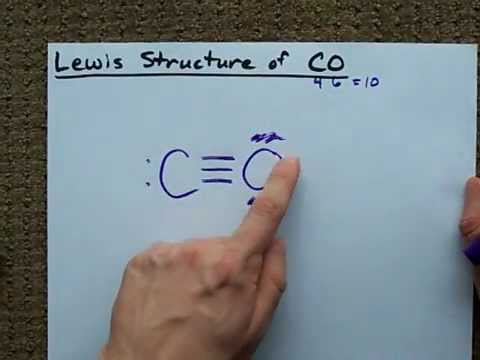
This gives us the following structure: Lewis structure of carbon monoxide CO (step 3 draw single bonds) Step 4: Place the remaining electrons on the atomsĪfter connecting the atoms with single bonds, we need to place the remaining electrons (10 – 2 = 8) on the atoms to satisfy the octet rule. Since carbon monoxide only has two atoms, both atoms are connected to each other with one bond ( 2 electrons). Next, we need to connect the atoms with single bonds. Step 3: Connect the atoms with single bonds There is no central atom because the molecule is diatomic. Since there are one carbon and one oxygen atom in carbon monoxide, the total number of valence electrons is:ġ×(4) + 1×(6) = 10 Step 2: Determine the central atom To determine the total number of valence electrons in carbon monoxide, we add up the valence electrons of each atom in the molecule.Ĭarbon (C) has 4 valence electron, and oxygen (O) has 6 valence electrons. Valence electrons are the electrons in the outermost energy level of an atom that participate in chemical bonding.
The first step in drawing the Lewis structure of carbon monoxide is to determine the total number of valence electrons present in the molecule.

Step 1: Determine the total number of valence electrons
#CO CARBON MONOXIDE LEWIS STRUCTURE HOW TO#
How to Draw the Lewis Dot Structure for Carbon monoxide We will explain step-by-step how to draw the dots of CO Lewis structure (carbon monoxide). These diagrams are a helpful tool in chemistry to understand the bonding behavior of atoms and how they interact with each other. Lewis structures are diagrams that show ho w atoms in a molecule are arranged and bonded to each other. What is the Lewis structure of carbon monoxide CO?


 0 kommentar(er)
0 kommentar(er)
